Although the entire information for the precise three dimensional structure of a protein is encoded in its amino acid sequence, in vivo many proteins depend on the assistance by molecular chaperones such as Hsp70 (DnaK) and Hsp60 (GroEL) heat shock proteins for folding from nascent or denatured state into their correct structure. It is known that in Escherichia coli the DnaK, DnaJ and GrpE chaperone machinery can efficiently repair misfolded Photinus pyralis luciferase both in vivo and in vitro but cannot protect it from heat induced unfolding. Experimental findings suggest that this refolding process is achieved by ATP-dependent interaction between the DnaK chaperone and the substrate protein or peptides. DnaJ and GrpE function as regulators in this system by stimulating DnaK's ATP hydrolysis activity and subsequent nucleotide exchange.
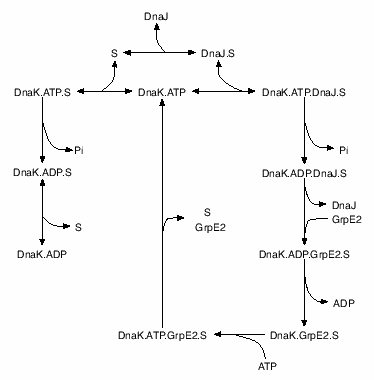
The kinetics of DnaK chaperone system has been studied extensively. Different mechanisms have been suggested to explain the steps in chaperone action. The mechanism suggested by Schröder et al has been widely accepted. In this proposed mechanism, an unfolded protein substrate (e.g. Photinus pyralis luciferase) first associates with DnaJ, which will present it to DnaK.ATP and induce the formation of a trimeric DnaK.ATP.DnaJ.substrate complex. DnaJ and substrate synergistically stimulate ATP hydrolysis by DnaK and thereby trigger the transition of DnaK form the ATP state with low affinities for substrates to the high-affinity ADP state. GrpE will bind to the latter complex and catalyze the release of ADP. Subsequent ATP binding induces conformational changes in the ATPase domain and substrate binding domain leading to a rapid dissociation of GrpE and substrate from the complex. These steps form a cycle of the DnaK-assistant folding. With enough ATP and all the chaperone molecules, after many cycles, the substrate can be refolded back to its active state see also. Here we describe a kinetic model for DnaK chaperone action in protein refolding based on Figure 1. The rate constants were derived from literature or completed by our experiments. Our model is shown to simulate correctly the behavior of E. coli DnaK chaperone action. The kinetic partition hypothesis proposed for protein refolding, sensitivity of refolding productivity to alterations in activity and concentration of the chaperones and ATP consumption are discussed.

Figure 1: Kinetic model of DnaK chaperone system in protein refolding