Robustness can be defined as the insensitivity to changes in variables. Here we tested the robustness of the DnaK chaperone system by reducing the initial values for the numbers of DnaK, DnaJ and GrpE molecules by half. We also found that after 50% reduction of the amounts of these chaperone molecules, the system is still able to maintain its behavior for refolding the substrate, which indicates that the chaperone system is robustly designed. Among the 3 chaperone molecules tested, a 50% reduction of DnaJ and GrpE had the strongest impact on the refolding process and no significant differences could be found in the results when DnaK and ATP was varied. This is surprising and contrasts in vitro observations. We will discuss this matter below. Tracing the dynamic changes in DnaK.ADP.DnaJ.S shows that more DnaK.ADP.DnaJ.S was accumulated if the concentration of GrpE is lowered. It is interesting that DnaJ, although it is part of this complex, has less effect compared with GrpE. This also supports our previous conclusion that GrpE can be both a direct or indirect regulator of this chaperone system.
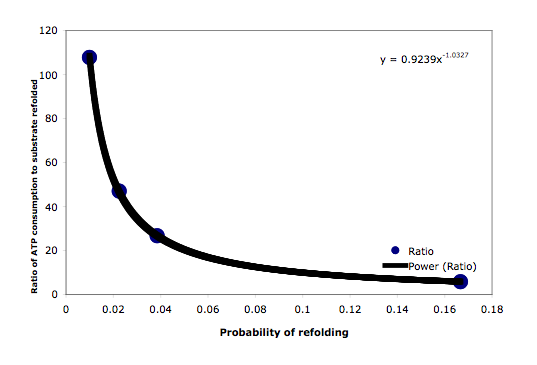
The function of DnaK chaperone system depends on the hydrolysis of ATP (16). In each cycle of the DnaK chaperone action, 1 molecule of ATP is hydrolyzed to drive the cycle and/or to provide the energy for the refolding process (Figure ). During the heat shock response, many cellular proteins will become unfolded and proteins belonging to different functional and structural groups will be affected. It is very likely that these proteins, when exposed to the DnaK machinery, require different numbers of chaperone cycles until they reach their native state. To survive heat shock, bacteria must refold as many proteins to their physiological state and as fast as possible. It is known that to achieve this, bacteria will accelerate the heat shock gene expression (17). The increasing repair activity concomitantly increases the ATP consumption (1 ATP per Hsp70 cycle, 7 ATP per GroEL cycle). However, some of the heat-inactivated proteins may be components of the energy generating systems. Thus it is an important question whether the ATP levels are sufficiently high to sustain the repair function. We addressed this question by using 4 different refolding probabilities and compare the ATP consumption. As summarized in Figure 3.4, the relationship between the probabilities of refolding and ATP consumption is nonlinear. A simple power fitting results an equation: y = 0.9239 x Ð1.0327. Therefore the refolding of proteins with a lower refolding probability after release by DnaK consumes much more ATP than the refolding of proteins with a higher refolding probability, especially if the refolding probability is less than about 5%, which means about 20 cycles are needed on average for the unfolded protein to return to its physiological conformation. For a protein substrate with only 1% probability to return to its native state after finishing a single chaperoning cycle, it will take more than 100 times the number of ATP molecules as compared to the number of denatured substrate molecules. Under such conditions, cellular ATP levels may soon be exhausted and the functionality of the heat shock proteins will be limited by the quantity of ATP. ATP generation will most likely decrease with increasing temperatures above the physiologically range for which the organism adapted. At the same time the unfolding probability for native proteins will increase and the refolding probability of proteins released after chaperoning by Hsp70 will decrease. Therefore slowing down the ATP consuming chaperone cycle by decreasing the activity of GrpE may be an evolutionary strategy to cope with such situations Taken together we concluded that although the DnaK chaperone system is robustly designed, this robustness is limited by the cellular amount of ATP.

Figure 6: ATP consumption and the probability of refolding
After more than 95were calculated and divided by the number of transformed substrates. Probability is the probability value of one substrate molecule after finishing exact one cycle of the chaperone system to be able to fold to natural states