

Next: Bibliography
Up: Results
Previous: Results
Both the R form or the T form of DnaK are known to have affinity to bind
with abnormal peptide and proteins, but the contribution of each form is
still unknown. To address this question, we modeled how HSP70 molecular
chaperon works with peptide using data from
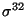 -Q132-Q144-C-IAANS
, a 12 amino acid residue peptide. This model indicated that when 6000 molecules of this
peptide were added to the model system at physiological steady state, about
4000 molecules will bind with DnaK.ATP in less than 1 second on average (Fig
3). And to our surprise that no DnaK.ADP can bind with this
peptide. To test whether this was due to the simulation time is not longer
enough, we run the model up to 1000 seconds, and still no DnaK.ADP.peptide
complex was found (data not shown).
-Q132-Q144-C-IAANS
, a 12 amino acid residue peptide. This model indicated that when 6000 molecules of this
peptide were added to the model system at physiological steady state, about
4000 molecules will bind with DnaK.ATP in less than 1 second on average (Fig
3). And to our surprise that no DnaK.ADP can bind with this
peptide. To test whether this was due to the simulation time is not longer
enough, we run the model up to 1000 seconds, and still no DnaK.ADP.peptide
complex was found (data not shown).
Figure 3:
DnaK binds peptide
|
|
Bin Hu
2004-02-25